- +91 22 3512 8738
- info@innvoceptsolutions.com
- Solus, 1002 & 1913, Thane, India 400607
Our Solutions
- Home
- Our Solutions
We provide comprehensive support for all aspects of the clinical research project. From study design and protocol development to regulatory compliance and data management, our team of experts shall work with you every step of the way to ensure the success of your trial.
Our Offerings
We are dedicated to providing personalized support and guidance every step of the way, to ensure that your trial meets all regulatory requirements and achieves the desired outcomes.
By exploring our solutions and offerings for clinical trial projects, you will have the opportunity to learn about the various services we provide and how we can support you in your research endeavor.
Regulated Clinical Studies
Ensuring medical products are safe and effective through clinical studies in compliance with regulatory guidelines.
Market Research & Analytics
Gaining insight into market trends, customers, competitors, and industry dynamics to inform business strategy through research.
Real-world Evidence Studies
Assessing the effectiveness, safety, and value of medical products through observational research in real-life settings.
Clinical Studies for Cosmetics
Conducting studies to evaluate safety and efficacy and support product development and regulatory compliance.
Digital Tools for Clinical Studies
Optimizing clinical studies through advanced digital technologies and tools, from e-consent to real-time data monitoring.
IGS Study
Our Study List
Here’s an overview of our current clinical research projects, including their therapeutic area, status, and key findings, to give stakeholders an insight into the organization’s ongoing work.
Based on ongoing & completed studies as of January 2023
Therapeutic area
Study Categories
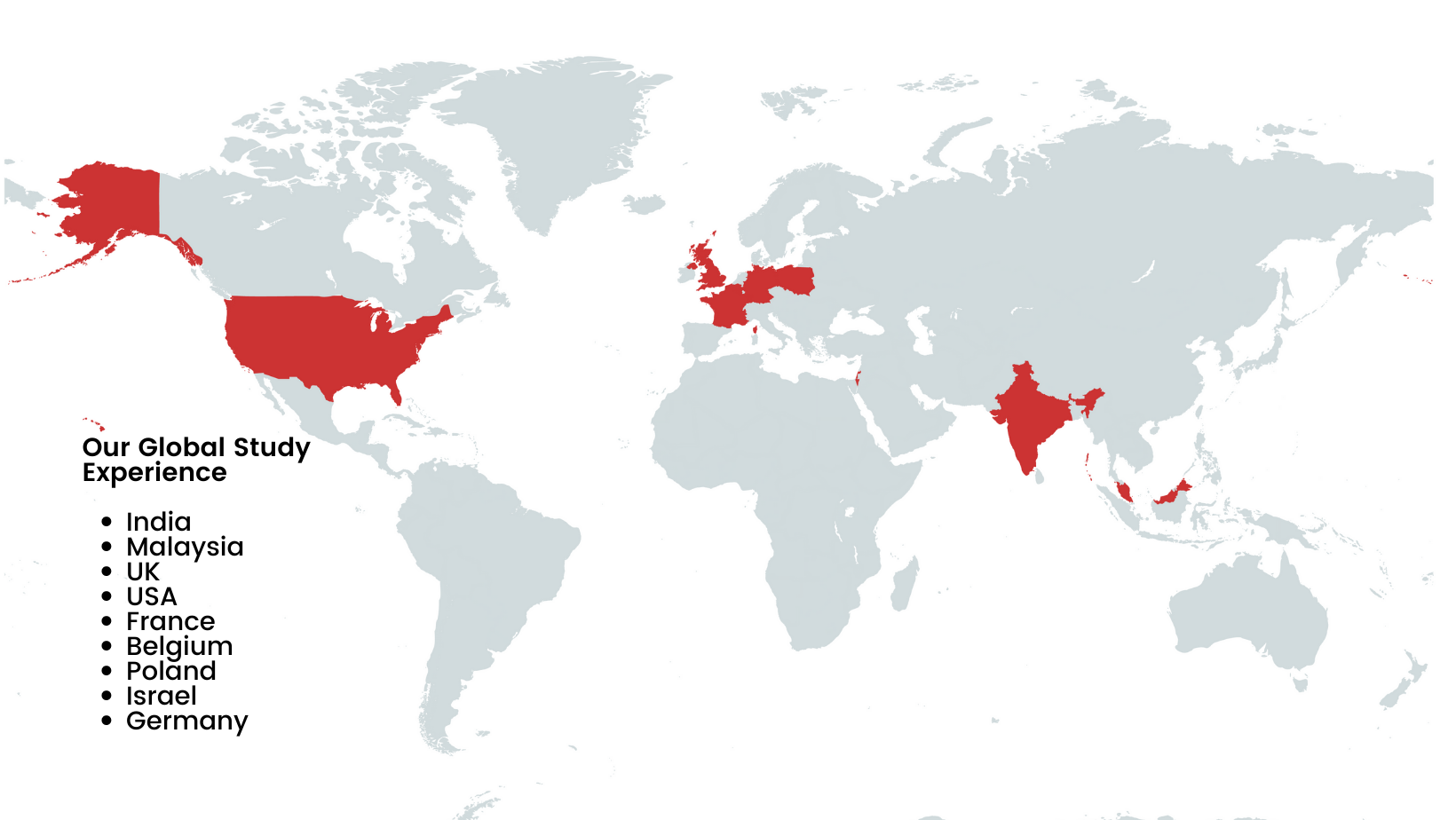
Our Global Footprint
Innvocept Global Solutions offers a wide range of clinical trial studies at various locations worldwide. We have a broad network of investigators and research sites across multiple therapeutic areas and geographies to conduct studies efficiently and effectively. Our study list is regularly updated, so you can stay informed about our ongoing and upcoming clinical trials at various locations.
Join our clinical trial journey today!

