Real World Data (RWD)/Real World Evidence (RWE) with possible solutions in India
Innvocept
July 15, 2022

Real World Data (RWD)/ Real World Evidence (RWE)
According to US FDA, real-world evidence (RWE) is clinical evidence regarding the usage, potential benefits, or risks of a medical product derived from an analysis of real-world data. Real-world evidence (RWE) trials are crucial for further understanding newly approved medicinal products in the real-world setting. These trials provide clinical evidence of long-term safety and efficacy. This article explains details about RWE studies, its challenges to perform in India, with possible solutions.
Introduction
Real World Evidence (RWE) is derived from Real World Data (RWD) that is collected from various sources such as electronic medical records, pragmatic clinical trials, prospective observational/ longitudinal cohort studies, retrospective databases, claims databases, patient and caregiver surveys, patient-generated data, including from in-home‑use settings, data gathered from other sources that can inform on health status, such as mobile devices. RWD is the information collected during routine clinical practice. (1)
There is a need to produce evidence in addition to RCTs (Randomized Clinical Trials), which is possible with RWD that generates answers to questions or helps test hypotheses, provides evidence on patient populations’ diseases, medicines, and health care that could help in clinical practice and also produces additional research questions. (2)
FDA uses RWE in monitoring post-marketing safety and also in making regulatory decisions. The health care providers use this data in developing guidelines for use in clinical practice. (3)
RWE and RCTs
RCTs are conducted for evaluating the safety and efficacy while RWE demonstrates the effectiveness of a drug or treatment pattern. The act of randomization in RCTs balances the participant characteristics between the groups, thus reducing bias in examining the cause-effect relationships between intervention and outcome. (4)
RCTs are conducted on selective populations and in ideal settings with set inclusion and exclusion criteria, due to which there is a chance that the population characteristics might fail to reflect the actual clinical site appropriately to represent the entire population. (5)
In simple words, the data from RCTs sometimes are not generalizable on the entire population given the varied disease comorbidities, ethnic differences, concomitant medications, differences in lifestyle, and vulnerable populations in routine clinical settings. (1)
Hence, RCTs require support from varied situations, and this is where RWE complements RCTs. (1,5,6,7)
RWE studies consume less time, cost, and resources compared to RCTs. Unlike RCTs which are complex and time taking in data accessibility and retrieval, RWE studies have the advantage of rapid and easy data retrieval and accessibility. Safe research on high-risk groups is not possible with RCTs which is possible in RWE studies. RCTs are conducted in smaller populations and for a shorter duration, which is not the case in RWE studies, thus RWE produces better results in the detection of less frequent side effects. (8)
Challenges in the use of RWD for RWE
Data Quality
Vigorous quality assurance processes are needed for sourcing the evidence from RWD since low-quality patient registries might lead to concerns from stakeholders. (9) Incomplete or missing data also might lead to the low data quality of RWD and certain sources of RWD are susceptible to data omissions leading to gaps, e.g., Claims data might show the tests undergone by the patient while not producing the details of the test results. Also, claims databases might not contain information on patients’ lifestyles and the severity of diseases. (10, 16)Standardization
From the design of principles, and conduct of data collection, processing, and reporting, there is a large gap between institutions reducing the quality of RWD when compared to data from RCTs. This shows that there is a requirement for data standardization. This can increase the use of RWD and RWE in drug development and the regulatory cycle. (11-16)Coordination
There is a requirement for coordination between organizations on the national and international level on RWE from RWD which might help in producing adequate evidence from limited research. (14, 16)Governance
Not all stakeholders have access to RWD which is due to varied sources of RWD such as academic institutions, and hospitals, and this shows that there is a need for legal frameworks and governance arrangements that would allow access to RWD to different groups and would help in utilizing the same in optimizing healthcare for patients in time. (11, 16)Data Validation
The validity is questionable since the RWE study designs are unblinded and non-randomized. (17)Confounding
In RWE studies, sometimes small observed effects might be due to confounding factors. (17)RWE Studies in India
A huge time and effort are needed to record patient data in a hospital. In India, it is very difficult to collate hospital data on standard indicators of the quality of patient care. Since there is no legislative framework or guidelines available in this country. Detailed records of patient history and information about treatment provided are either provided very less or remain unavailable in the majority of hospitals. To yield data that can be analyzed appropriately, well-defined formats whether in the physician’s clinic or hospitals are required for RWD. (6)
In India, although there are many RWE studies conducted by different pharmaceutical companies in private clinics and hospitals, very few numbers of studies are featured in a public forum like the Clinical Trials Registry of India (CTRI). These studies are not accessible to the public easily. The below graph explains the registration of RWE studies between Academic investigators & Pharma Industries as per the analysis of CTRI, since its 2009 inception. (18)
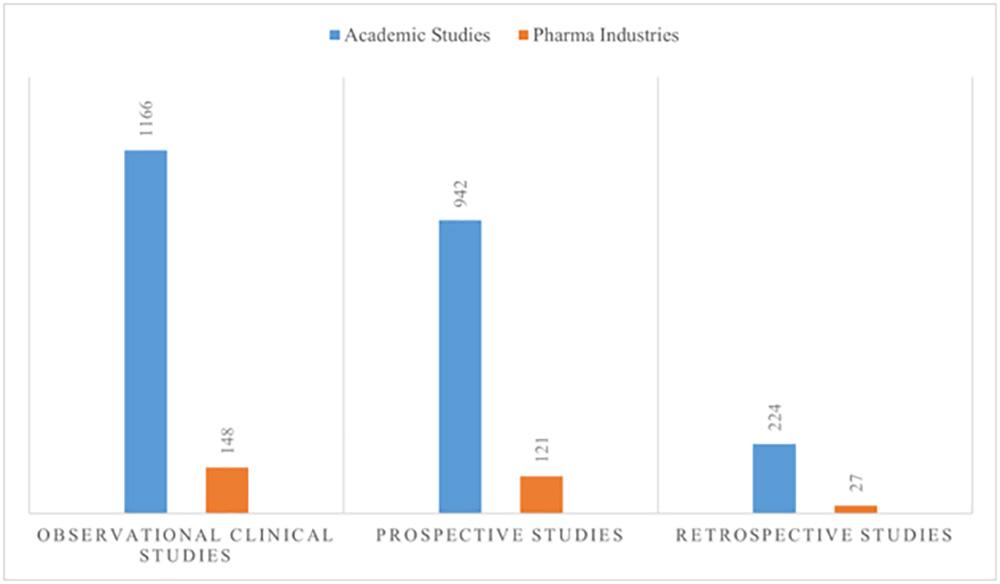
The therapeutic areas of these observational studies are majorly on different types of Cancer followed by cardiovascular disease and Diabetes. (19)
Most of the studies conducted were not systematically planned RWE studies with considerations of design, validity, and quality except for the studies conducted by Academic Investigators.
The above findings suggest that there is a lack of good-quality clinical records for RWE studies. However, this finding also indicates a large number of non-industry studies, which highlights academic clinical investigator interest in conducting observational research studies. This can be an opportunity for the industry to utilize the tremendous potential of such academic studies. (18)
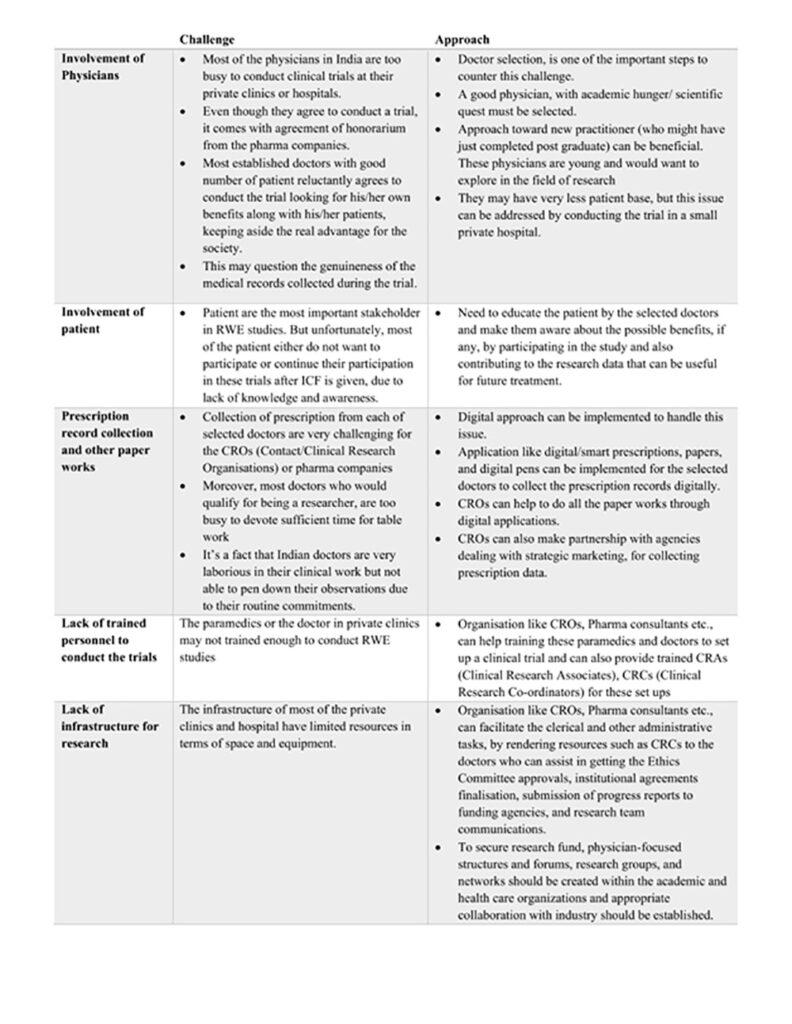
Challenges v/s Approaches in conducting RWE studies at private clinics and hospitals- Indian Prospective (20, 21)
Mitigation by Regulatory Authorities (22, 23)
- A study implementation template should be developed. This template will give an idea about methods-related items from existing checklists corresponding to the main headings in structured tables where critical details are communicated.
- A template for planning and reporting on RWE study implementation needs to be developed.
- Development of a Natural Language Processing (NLP) Web Service for Public Health is of great use. NLP allows computers and other technologies to interpret human language. This removes the manual process for researchers while increasing the completeness, timeliness, and accuracy of narrative text data, which can then be used for public health research.
- Patient-Generated and Electronic Health Record (EHR) Data should be linked.
- Linking of EHR Data to Other RWD Sources should also be considered.
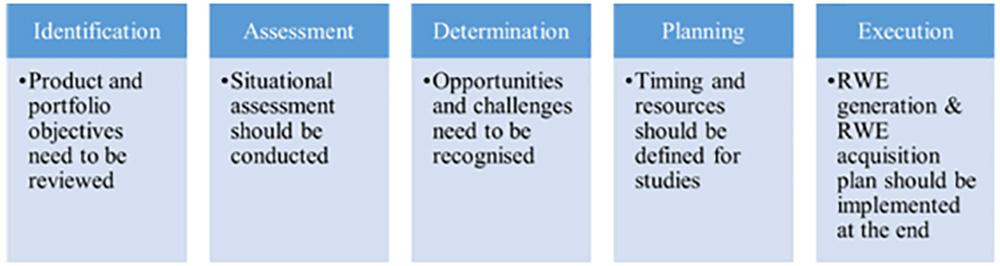
Overview of RWE planning (24)
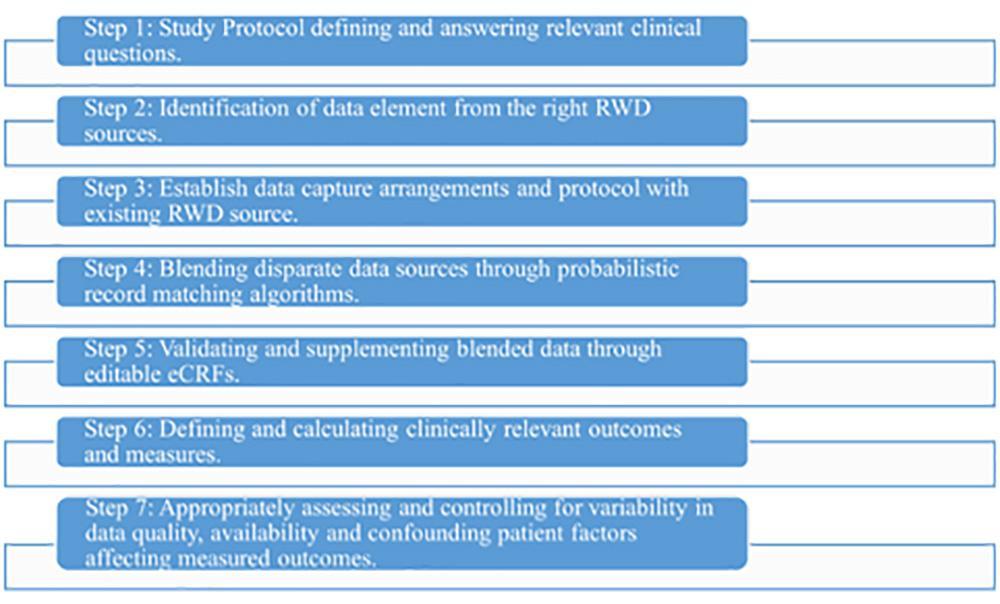
Steps to create RWE (25)
Insights of RWE (26)
- Modern Patient Registries
- Value-based reimbursements
- Key Opinion Leader (KOL) engagement
- Patient journey
- Treatment pathways
- Payers insight
- Market analysis
- Artificial Intelligence/Machine Learning (AI/ML) based literature review
References
- Poobalan Naidoo, Célia Bouharati, Virendra Rambiritch, Nadina Jose, Sumanth Karamchand, Robert Chilton, Rory Leisegang, Real-world evidence, and product development: Opportunities, challenges and risk mitigation, Springer-Verlag GmbH Austria, part of Springer Nature 2021.
- Simon de Lusignan, Laura Crawford, Neil Munro, Creating and using real-world evidence to answer questions about clinical effectiveness. J Innov Health Inform. 2015;22(3):368-373.
- Real-World Evidence | FDA
- Eduardo Hariton, Randomised controlled trials—the gold standard for effectiveness research, HHS Public Access, Author manuscript, BJOG. Author manuscript; available in PMC 2018 December 01.
- Hun-Sung Kim, Suehyun Lee, Ju Han Kim, Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records, Korean Med Sci. 2018 Aug 20;33(34).
- Chodankar D. Introduction to real-world evidence studies. Perspect Clin Res 2021; 12:171-4.
- N.Wierzbicka, Janssen, K. Jahnz-Różyk, The evolving landscape for Real World Evidence in Poland: physicians’ perspective, Journal of Health policy and outcomes research, 2015, 1, 15-33.
- Cole A, Garrison L, Mestre-Ferrandiz J, Towse A. Data governance arrangements for real-world evidence; 2015. Available from: https://www.ohe.org/publications/data-governance-arrangements-real-world-evidence.
- Makady A, de Boer A, Hillege H, Klungel O, Goettsch W. What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health. 2015 Jul 1;20(7):858–65.
- Miani C, Robin E, Horvath V, Manville C, Cave J, Chataway J. Health and healthcare: assessing the real world data policy landscape in Europe. Rand Health Q. 2014;4(2):15.
- Hampson G, Towse A, Director M, Dreitlein B, Henshall C. Real-world evidence for coverage decisions: opportunities and challenges [Internet]; 2018. Available from: https://icer-review. org/wp-content/uploads/2018/03/ICER-RealWorld-Evidence-White-Paper-03282018.pdf.
- Wise J, Möller A, Christie D, Kalra D, Brodsky E, Georgieva E, et al. The positive impacts of real-world data on the challenges facing the evolution of biopharma. Drug Discov Today. 2018;23(4):788–801.
- Sun X, Tan J, Tang L, Guo JJ, Li X. Real-world evidence: experience and lessons from China. BMJ. 2018;360:j5262.
- European Medicines Agency. RD-ACTION, European medicines agency, and European commission-DG SANTE workshop: how European Reference Networks can add value to clinical research the EMA patient registries initiative [Internet]; 2018. Available from: https://www. ema. europa.EU/en/documents/presentation/ presentation-European-medicines-agency-patient-registries-initiative-Xavier-kurz_en.pdf
- Program. FDA Framew. 2018. Available from: https: //www.fda.gov/media/120060/download.
- Frank Grimberg, Petra Maria Asprion, Bettina Schneider, Enkelejda Miho, Lmar Babrak, Ali Habbabeh, The Real-World Data Challenges Radar: A Review on the Challenges and Risks regarding the Use of Real-World Data, Digit Biomark 2021;5:148–157.
- Bhatt A. Conducting real-world evidence studies in India. Perspectives in Clinical Research 2019; 10(2); 51-56.
- https://globalforum.diaglobal.org/issue/november-2020/enabling-rwe-studies-in-india/. Accessed on 23.9.21.
- http://ctri.nic.in/Clinicaltrials/advancesearchmain.php. Accessed on 23.9.21.
- Rahman S, Mazumdar Md Anwarul A, Shaban Shami F, et al. Physician participation in clinical research and trials: issues and approaches. Advances in Medical Education and Practice 2011; 2: 85–93.
- Agarwal PK. Clinical trials in private clinics. Perspect Clin Res 2011; 2:90-3.
- Generating Real-World Evidence by Strengthening Real-World Data Sources. Department of Health and Human Services, USA;1-6.
- Wang Shirley V, Pinheiro Simone, Hua Wei, et al. STaRT-RWE: structured template for planning and reporting on the implementation of real-world evidence studies. BMJ 2021; 1-8.
- https://iconplc.com/services/real-world-intelligence/rwe-strategy-analytics/index.xml. Accessed on 23.9.21.
- https://www.arbormetrix.com/blog/9-ways-real-world-evidence-is-changing-healthcare. Accessed on 23.9.21.
- https://www.saama.com/wp-content/uploads/2020/02/Saama_ICON_Factsheet_Real_World_Evidence_Platform.pdf
Recent Post
-
10 Jul 2023Unveiling the Power of RWE Clinical Trials: India's Advantages and Emerging Trends in Pharma Industry
-
14 Aug 2022Trends in store for the clinical and contract research organizations post-pandemic
-
15 Jul 2022Real World Data (RWD)/Real World Evidence (RWE) with possible solutions in India



